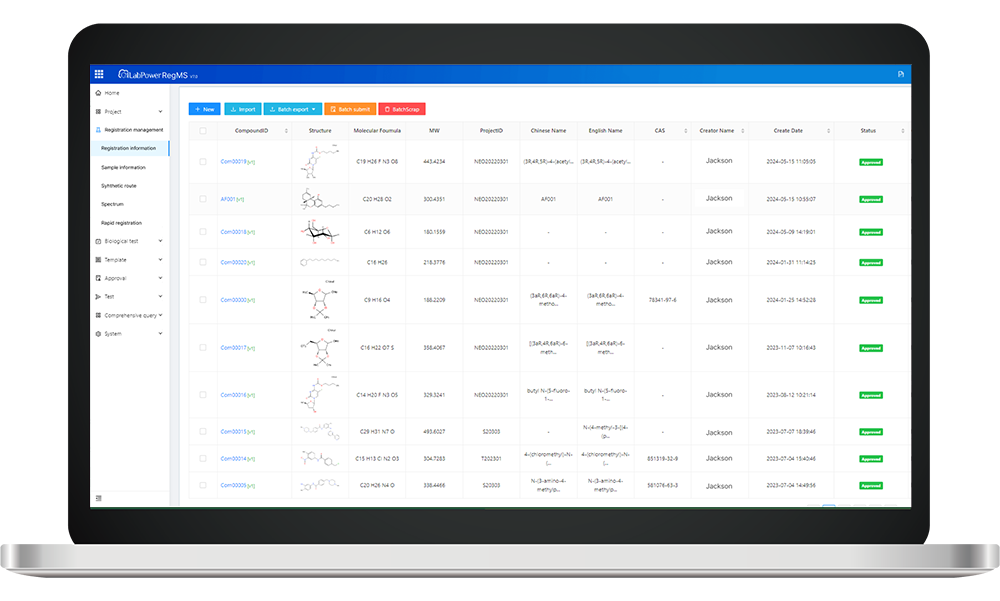
In recent years, the global market for monoclonal antibodies has surged to an astonishing $150 billion, underscoring their critical role in therapeutic applications. As this sector continues to expand, it becomes increasingly essential to establish a robust antibody Registration system that adheres to legal and regulatory frameworks.
An Overview of the Antibody Registration System and Its Legal Characteristics
The antibody registration system serves as a vital mechanism for ensuring that antibodies are developed, tested, and marketed in compliance with established laws and regulations. This system is characterized by its stringent requirements for documentation, safety assessments, and efficacy evaluations. Furthermore, ethical standards play a pivotal role in guiding research practices within this framework; they ensure that all studies involving human subjects or animal models adhere to principles of respect, beneficence, and justice.
Molecular Modeling and Simulation: Ethical Standards in Focus
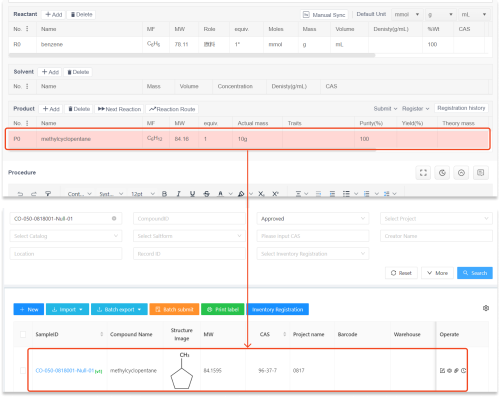
molecular modeling and simulation techniques have emerged as indispensable tools within the antibody development process. These methodologies not only facilitate predictive analyses but also align closely with ethical standards by minimizing unnecessary experimentation on live subjects. By employing computational approaches early in drug design phases, researchers can optimize candidate selection while adhering to guidelines aimed at reducing harm—thus promoting responsible scientific inquiry.
Characteristics of Neotrident Within Ethical Standards Frameworks
- Transparency: Neotrident emphasizes clear communication regarding data sharing practices related to antibody research.
- Accountability: The platform holds researchers accountable for maintaining high ethical standards throughout their work processes.
- Sustainability: Neotrident promotes sustainable practices by encouraging methods that reduce environmental impact during antibody production.
- Diversity Inclusion: The initiative advocates for diverse representation within clinical trials associated with new antibodies.
- User-Centric Design: It prioritizes user engagement through feedback mechanisms designed to enhance ethical compliance among stakeholders involved in antibody development.
A Conclusion on Antibody Registration Systems’ Ethical Implications
The exploration of the antibody registration system reveals its integral relationship with legal frameworks governing biomedical research. Through adherence to ethical standards such as transparency, accountability, sustainability, diversity inclusion, and user-centric design exemplified by initiatives like Neotrident—the field can advance responsibly while safeguarding public trust. In summary, establishing comprehensive regulatory attributes is paramount not only for innovation but also for upholding societal values inherent in scientific progress.